Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
1.
A Carnot engine is working between 127°C and 27°C. The increase in efficiency will be maximum when the temperature of
(a) the source is increased by 50°C
(b) the sink is decreased by 50°C
(c) source is increased by 25°C and that of sink is decreased by 25°C
(d) both source and sink are decreased by 25°C each
Ans (b)
2.
Three moles of an ideal monoatomic gas undergoes the process ABCDA as shown, where {{T}_{A}}\ =\ 300K, {{T}_{B}}\ =\ 600K, {{T}_{c}}\ =\ 1800\ Kand {{T}_{D}}\ =\ 900\ K. Find the work done by the gas during the cycle.
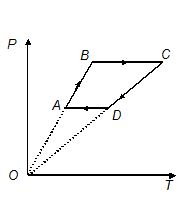
(a) 17.8 kJ
(b) 14.9 kJ
(c) 19.9 kJ
(d) 21.1 kJ
Ans (b)
3.
A thermo dynamical system goes from state (i) {{P}_{1}},\,Vto 2{{P}_{1}},\,V (ii) P, V to P, 2V. Then work done in the two cases is
(a) zero, zero
(b) zero, PV
(c) P{{V}_{1}}, zero
(d) P{{V}_{1}}, {{P}_{1}}{{V}_{1}}
Ans(b)
4.
An ideal gas is taken through series of changes represented in fig. The net work done by the gas at the end of the cycle is equal to
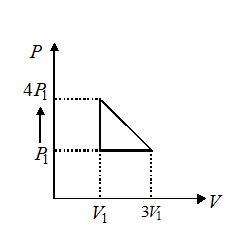
(a) {{P}_{1}}{{V}_{1}}
(b) 3{{P}_{1}}{{V}_{1}}
(c) 6{{P}_{1}}{{V}_{1}}
(d) 12{{P}_{1}}{{V}_{1}}
Ans (d)
5.
Heat energy absorbed by a system in going through a cyclic process shown in figure is

(a) {{10}^{7}}\pi J
(b) {{10}^{4}}\pi J
(c) {{10}^{2}}\pi J
(d) {{10}^{{-3}}}\pi J
Ans (c)
6.
An ideal gas is taken through the cycle A→B→C→A, as shown in the figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process C→A is
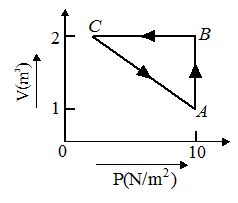
(a) –5 J
(b) –10 J
(c) –15J
(d) –20 J
Ans(a)
1.
A fixed mass of gas undergoes the cycle of changes represented by PQRSP as shown in figure. In some of the changes, work is done on the gas and in other; work is done by the gas. In which pair of the changes is work done on the gas?
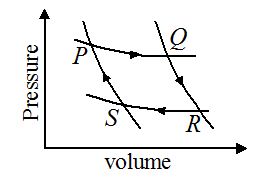
(a) PQ and RS
(b) QR and RS
(c) RS and SP
(d) PQ and QR
Ans (c)
2.
The first operation involved in a Carnot cycle is
(a) isothermal expansion
(b) adiabatic expansion
(c) isothermal compression
(d) adiabatic compression
Ans (a)
Get Full Access Of the Chapters
