Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
1.
A Carnot engine is working between 127°C and 27°C. The increase in efficiency will be maximum when the temperature of
(a) the source is increased by 50°C
(b) the sink is decreased by 50°C
(c) source is increased by 25°C and that of sink is decreased by 25°C
(d) both source and sink are decreased by 25°C each
Ans (b)
2.
Three moles of an ideal monoatomic gas undergoes the process ABCDA as shown, where {{T}_{A}}\ =\ 300K, {{T}_{B}}\ =\ 600K, {{T}_{c}}\ =\ 1800\ Kand {{T}_{D}}\ =\ 900\ K. Find the work done by the gas during the cycle.
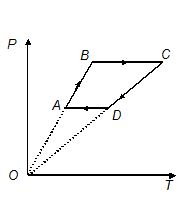
(a) 17.8 kJ
(b) 14.9 kJ
(c) 19.9 kJ
(d) 21.1 kJ
Ans (b)
3.
A thermo dynamical system goes from state (i) {{P}_{1}},\,Vto 2{{P}_{1}},\,V (ii) P, V to P, 2V. Then work done in the two cases is
(a) zero, zero
(b) zero, PV
(c) P{{V}_{1}}, zero
(d) P{{V}_{1}}, {{P}_{1}}{{V}_{1}}
Ans(b)
4.
An ideal gas is taken through series of changes represented in fig. The net work done by the gas at the end of the cycle is equal to
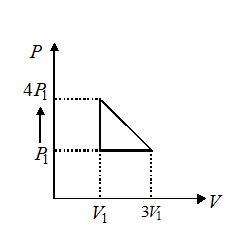
(a) {{P}_{1}}{{V}_{1}}
(b) 3{{P}_{1}}{{V}_{1}}
(c) 6{{P}_{1}}{{V}_{1}}
(d) 12{{P}_{1}}{{V}_{1}}
Ans (d)
5.
Heat energy absorbed by a system in going through a cyclic process shown in figure is

(a) {{10}^{7}}\pi J
(b) {{10}^{4}}\pi J
(c) {{10}^{2}}\pi J
(d) {{10}^{{-3}}}\pi J
Ans (c)
6.
An ideal gas is taken through the cycle A→B→C→A, as shown in the figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process C→A is
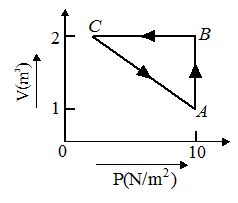
(a) –5 J
(b) –10 J
(c) –15J
(d) –20 J
Ans(a)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Get Full Access Of the Chapters
