Video Lecture
Theory For Making Notes
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Practice Questions (Level-1)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Practice Questions (Level-2)
1.
A ball is heated upto temperature T. The numerical value of T is maximum on which of the following scale
(a) Celsius
(b) Kelvin
(c) Fahrenheit
(d) Any of the above
Ans (b)
2.
The value of the gas constant (R) calculated from the perfect gas equation is 8.32\,J\,mo{{l}^{{-1\,}}}{{K}^{{-1}}}, whereas its value calculated from the knowledge of {{C}_{p}} and {{C}_{v}} of the gas is 1.98 cal\,\,mo{{l}^{{-1\,\,}}}{{K}^{{-1}}}. From this data, the value of J is
(a) 4.16 J/cal
(b) 4.18 J/cal
(c) 4.20 J/cal
(d) 4.22 J/cal
Ans (c)
3.
Temperature of gas is a measure of
(a) the average transnational kinetic energy of the gas molecules
(b) the average potential energy of the gas molecules
(c) the average distance of the gas molecules
(d) the size of the gas molecules
Ans (a)
4.
The absolute zero is the temperature at which
(a) water freezes
(b) all substances exist in solid state
(c) molecular motion ceases
(d) none of the above
Ans (c)
5.
Maximum density of water is at the temperature
(a) 32°F
(b) 39.2°F
(c) 42°F
(d) 4°F
Ans (b)
6.
Sea animals are safe in winter in cold countries because of
(a) their body conditions
(b) high specific heat of water
(c) low conductivity of water
(d) anomalous expansion of water
Ans (d)
7.
The temperature of the sun is measured with
(a) platinum thermometer
(b) gas thermometer
(c) pyrometer
(d) vapour pressure thermometer
Ans (c)
8.
We write the relation for Boyle’s law in the form PV = C, when the temperature remains constant. In this equation, C depends upon
(a) the quantity of the gas enclosed
(b) the atmospheric pressure
(c) the magnitude of in the laboratory
(d) the nature of the gas used in the experiment
Ans (a)
9.
From the set of lines in the Figure, choose the correct graph that gives the Fahrenheit temperature against the corresponding Celsius temperature.
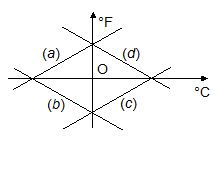
(a) a
(b) b
(c) c
(d) d
Ans (a)
9.
The \frac{{{{C}_{p}}}}{{{{C}_{v}}}} ratio of a gas mixture consisting of 8 g of helium and 16 g of oxygen is
(a) 1.33
(b) 1.47
(c) 1.59
(d) 2
Ans (c)
10.
If for a gas \frac{R}{{{{C}_{v}}}}=0.67, the gas is made of molecules which are
(a) diatomic
(b) mixture of diatomic and polyatomic molecules
(c) monoatomic
(d) polyatomic
Ans (c)
11.
420 J of heat is used in heating 10 g of water. The temperature of water then increases by
(a) 10°C
(b) 4.2°C
(c) 42°C
(d) 100°C
Ans (a)
12.
300 gram of water at 25°C is added to 100 gram of ice at 0°C. The final temperature of the mixture is
(a) 17.3°C
(b) 18.75°C
(c) 1.25°C
(d) 0°C
Ans (d)
13.
The average translation kinetic energy per gram mole of a gas is given by
(a) (3/2) RT
(b) (1/2) RT
(c) (2/3) RT
(d) (1/3 RT)
Ans (a)
14.
A gas occupies 1.5 m3 at 0°C. If the temperature is raised to 273° C without altering the pressure, the new volume of the gas will be
(a) 1.5 m3
(b) 2 m3
(c) 3 m3
(d) 0.75 m3
Ans (c)
15.
A given mass of an ideal gas with \displaystyle \gamma = 1.4 has volume V and pressure P. It is now compressed adiabatically to 1/32 times its original volume. The final pressure of the gas is
(a) 128P
(b) 64 P
(c) 32 P
(d) 256 P
Ans (a)
16.
The temperature at which the rms velocity of a hydrogen molecule is double that of an oxygen molecule at {{27}^{{}^\circ }}C is?
(a) 1200 K
(b) 75 K
(c) 150 K
(d) 600 K
Ans (b)
17.
The temperature of a gas increases by 10°C. What is the equivalent increase on the Kelvin scale?
(a) 10 K
(b) 0 K
(c) 100 K
(d) 273.90 K
Ans (a)
18.
The readings of air thermometer at 0°C and 100°C are 50 cm and 75cm of mercury column respectively. The temperature at which its reading is 80 cm of mercury column is
(a) 105°6C
(b) 110°C
(c) 115°C
(d) 120°C
Ans (d)
19.
Two thermometers xand y have fundamental intervals of 80° and 120°. When immersed in ice, they show the readings of 20° and 30°. If y measures the temperature of a body as 120°, the reading of x is
(a) 55°
(b) 65°
(c) 75°
(d) 80°
Ans (d)
20.
A Fahrenheit thermometer reads 113° F while a faulty Celsius thermometer reads 44°C. The correction required to be applied to the Celsius thermometer is
(a) –1°C
(b) +1°C
(c) +2°C
(d) –2°C
Ans (b)
