Video Lecture
Theory For Making Notes
Radiation Pressure
Even though the rest mass of a photon is zero, it possess a linear momentum. When this photon falls on a surface it undergoes either reflection or absorption. During both the process there is a change in momentum of the photon and hence there exist a force.( Linear momentum cannot change without a force) Therefore whenever a radiation falls on a surface it experience a force and as a reaction it exert a force on the surface. The normal component of this force exerted per unit area of the surface is called the radiation pressure.
Momentum carried by photon
We know that wavelength of the radiations is given by
λ=ph …(i) where h is the Planck’s constant and p is the momentum of the photons.
Also we know that energy `E’ of a photon is given as
E=hν where frequency ν=λc
therefore E=λhc …(ii)
putting λ from equation (i) into equation (ii)
we get E=h/phc
⇒E=pc
hence p=cE This is the momentum carried by photon of energy E
Intensity of radiations
It is the energy passing ( or falling) per unit area per unit time through ( or on) a surface placed perpendicular to the path of the radiations.
So I=A.ΔtE , where E is energy , A is area placed perpendicular to the radiation and Δt is time for which energy passes .
Also we know that ΔtE=P (Power)
Hence I=AP
Calculation Of Radiation Pressure
CASE-1
When the radiations falls normally and fully (100%) absorbed by the surface

We know that the momentum carried by radiations is
p=cE … (iii) ,
where E is the energy incident on the surface through radiation in time Δt.
Also we have seen that the Intensity I is given by
I=A.ΔtE …(iv)
dividing (iii) by (iv) we get
Ip=cEEAΔt
⇒ p=cIAΔt …(v)
(in the above equation p is the initial momentum carried by radiations which fall on the surface for time \Deltat)
In case of 100% absorption of the radiations all this momentum becomes zero finally
hence final momentum p′=0
so change in momentum in time Δt is Δp
where Δp=p′−p=0– cIAΔt= –cIAΔt
Hence ∣Δp∣=cIAΔt
or ∣∣ΔtΔp∣∣=cIA
or F=cIA ∵ ∣∣ΔtΔp∣∣ = F (magnitude of force)
Here F is the force that developed due to change in momentum of the radiations as they are absorbed by the surface.
We can divide both sides by area `A’ we get AF=cI
Hence Radiation Pressure P(radiation) is given by Pradiation=cI (∵ AF = Pressure)
CASE-2
When the radiations falls normally and fully reflected by the surface i.e. 100% reflection

From equation no. (v) we can write the momentum of radiation before and after reflection
Initial momentum of radiations
p=cIAΔt
Final Momentum after reflection
p’=−cIAΔt
so change in momentum is Δp
where Δp=p′−p = −cIAΔt−cIAΔt= −c2IAΔt
Hence ∣Δp∣=c2IAΔt
or ∣∣ΔtΔp∣∣=c2IA
or F=c2IA ∵ ∣∣ΔtΔp∣∣ = F (magnitude of force)
Here F is the force that developed due to change in momentum of the radiations as they are reflected by the surface.
We can divide both sides by area `A’ we get AF=c2I
Hence Radiation Pressure P(radiation) is given by
Pradiation=c2I
(∵ AF = Pressure)
Note that Radiation pressure in case of 100% reflection is twice that in case of 100% absorption.
X-Rays
Production of X-rays
When highly energetic electrons are made to strike a metal target, electromagnetic radiations comes out. A large part of this radiations has wavelength of the order of 1Ao and is called X-rays.
Soft and hard X-ray – The X-rays of low wavelengths are called hard X-rays and large wave lengths are called soft X-rays hard and soft are simply relative terms.
The device which is used to produce X-rays is called Coolidge tube as shown below.
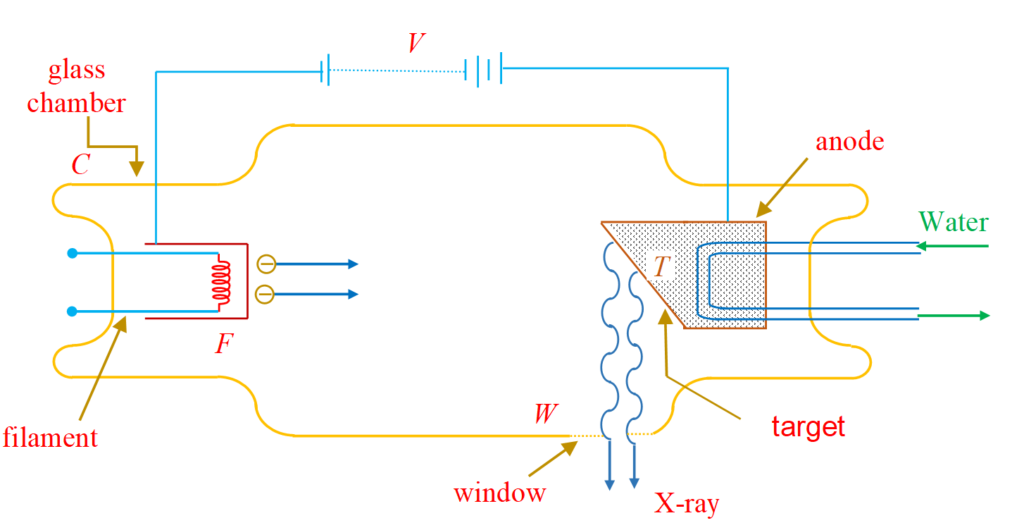
A filament F and a metallic target T are fixed in an evacuated glass chamber C. The filament is heated electrically and emits electrons by thermionic emission. A constant potential difference of several kilovolt is maintained between the filament and the target using a dc power supply so that the target is at a higher potential than the filament. The electrons emitted by the filament are, therefore, accelerated by the electric field set up between the filament and the target and hit the target with a very high speed. These electrons are stopped by the target and in the process X-rays are emitted. These X-rays are brought out of the tube through a window W made of thin mica or mylar or some such material which does not absorb X-rays appreciably. In this process, large amount of heat is developed, and thus an arrangement is provided to cool down the tube continuously by running water.
Continuous And Characteristic X-Rays
When X-rays coming from a Coolidge tube are investigated for the wavelength present. We find that X-rays can be divided in two categories based on the mechanism of their generation. These X-rays are called continuous and characteristic X-rays. These X-rays have their origin in the manner in which the highly energetic electron loses its kinetic energy. As the fast moving electrons enter metal target, they starts losing their energy by collisions with the atoms of metal target. At each such collision either of the following two processes take place.
(i)
Electron loses its kinetic energy and a part of this lost kinetic energy is converted into a photon of electromagnetic radiation and the rest increases the kinetic energy of the target atoms, which ultimately heats up the target. This electromagnetic radiation is nothing but continuous X-rays. The fraction of kinetic energy converting into energy of photon varies from one collision to the other and the energy of such photon will be maximum when electron converts all its energy into a photon in the first collision itself.
If electron are accelerated through a potential difference V, then maximum energy of emitted photon could be Emax = eV , λminhc = eV , λmin = eVhc
λmin is also called cut off wavelength. Since electron may loose very small energy in a given collision, the upper value of λ will approach to infinity. However both the cases e.g. electron converting all its energy in one will go and loosing very small energy will have very small probability. That explains the origin of continuous X-ray. We can also see from the discussion, which we have had on continuous X-rays that λmin depends only on accelerating voltage applied on the electron and not on the material of the target.
(ii)
The electron A knocks out an inner shell electron C of the atom with which it collides. Let us take a hypothetical case of a target atom whose K-shell electron has been knocked out as shown.
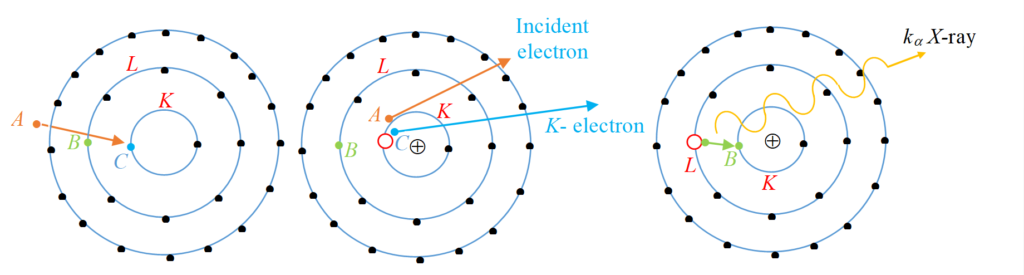
This will create a vacancy in K-shell shown by red circle. Sensing this vacancy an electron B from a higher energy state say L shell may make a transition to this vacant state. When such a transition takes place the difference of energy is converted into photon of electromagnetic radiation, which is called characteristic X-rays. Now depending upon which shell electron makes a transition to K-shell we may have different K X-rays e.g. if electron from L shell jumps to K shell we have Kα, if electron from M shell jumps to K shell, we have Kβ X-ray and so on. Similarly if vacancy has been caused in L shell we may have Lα, Lβ X-ray etc depending on whether we have transition of electron from M shell or N shell.
Below we have shown the production of characteristic x rays i.e. Kα,Kβ,… and Lα,Lβ,… on a energy level diagram.
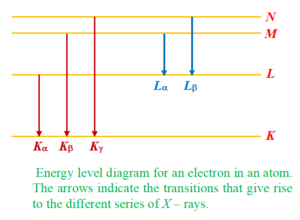
Since emission of characteristic X-ray involves the inner material energy levels of target atom, therefore the wavelength of characteristic X-ray depends on the target. If we plot curve between intensity of different wavelength components of X-ray coming out of a Coolidge tube and wavelength, it is like a figure given below:
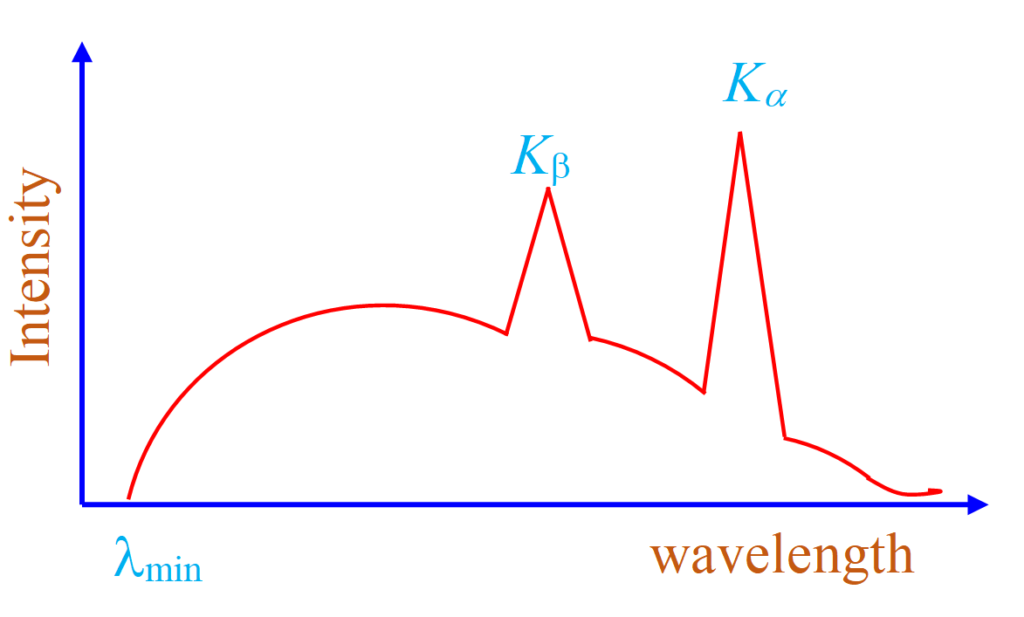
As we can see from the curve, at certain clearly defined wavelengths, the intensity of X-rays is very large. These X-rays are known as characteristic X-rays. It is also clear from the curve that λkβis greater than λkαhowever intensity of Ka transition is more as compared to the intensity of Kβ transition
It is primarily because transition probability of Kα is more as compared to transition probability of Kβ. At other wavelengths intensity varies gradually and these are called continuous X-rays.
Moseley’s law
Moseley conducted many experiments on characteristic X-rays, the findings of which played an important role in developing the concept of atomic number. Moseley’s observations can be expressed as f =a(Zb) ,where a and b are constants, Z is the atomic number of target atom and f is the frequency of characteristic X-rays.
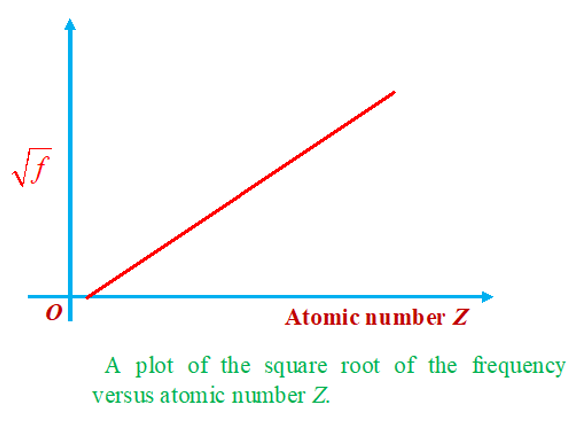
Moseley’s law can be easily understood on the basis of Bohr’s atomic model. Let us consider an atom from which an electron from K-shell has been knocked out, an L shell electron which is about to make transition to the vacant site will find the charge of nucleus is screened by the spherical cloud of remaining one electron in the K shell. If the effect of outer electrons and other L-electrons are neglected then electron making the transition will find a charge (Z – 1)e at the centre. Hence we may expect Bohr’s model to give expected result if we replace Z by (Z – 1).
According to Bohr’s model, the energy released during a transition from n = 2 to n = 1 is given by
ΔE=Rhc(Z1)2 (121−221)
hf = Rhc (43) (Z1)2
f = 43Rc (Z – 1)
Which is same as Moseley’s equation with b = 1 & a = 43Rc.
Properties of X-rays
(i) X-rays being an electromagnetic wave travel with a speed equal to the speed of light.
(ii) X-rays are not responsive to electric or magnetic field.
(iii) X-rays when pass through gases, produce ionisation.
(iv) X-rays can effect photographic plates and exhibit the phenomenon of fluorescence.
Illustration
Find the cut-off wavelength of the X-rays emitted by an X-ray tube operating at 30 kV.
Solution
For minimum wavelength, the total kinetic energy should be converted into an X-ray photon.
Thus,
λmin= Ehc=E12400=30×10312400 = 0.41 Å
Illustration
Show that the frequency of Kβ X-ray of a material equals to the sum of frequencies of Kα and Lα X-rays of the same material.
Solution

The energy level diagram of an atom with one electron knocked out is shown above.
Energy of Kα X-ray is
EKα=EL−EK
Energy of Kβ X-ray is
EKβ=EM−EK
and, Energy of Lα X-ray is
ELα=EM−EL
thus, EKβ=EKα+ELα
or fKβ=fKα+fLα
Illustration
If the shortest wavelengths of the continuous spectrum coming out of a Coolidge tube is 0.1Ao. Find the de Broglie wavelength of the electron reaching the target metal in the Coolidge tube (approximately) (given hc = 12400 eVAo, h = 6.63×1034 in MKS, mass of electron = 9.1×1031 kg)
Solution
Kinetic energy of the electron reaching the target metal = maximum kinetic energy of x-ray photon = 0.112400eV = 124000 eV = 124 x 1.6 x 10–16 J
de Broglie wavelength of the electron
λ=2mKh
hence λ=2×9.1×10−31×124×1.6×10−166.63×10−34
therefore λ=0.035Ao
Illustration
When the voltage applied to an X-ray tube is increased from 10 kV to 20 kV the wavelength interval between the Kα line and the short wave cut off of the continuous X-ray spectrum increases by a factor 3. Find the atomic number of element of which the tube anti-cathode is made. (Rydberg’s constant = 107 m–1)
Solution
λC1= eVhc=10×10312375A∘ = 1.2375 Ao … (1)
similarly λC2=0.61875 Ao … (2)
λKα1= (Z−1)2 {11−41}×107 … (3)
It is given
3 × (λKα – 1.2375) = λKα – 0.61875
hence λKα= 1.54338 Ao … (4)
Putting this value in (3)
Z –1 = 29
hence Z = 30
Illustration
A X-ray tube is operating at 12 kV and 5 mA. Calculate the number of electrons striking the target per second and the speed of striking electrons.
Solution
We know that I=tQ=tne
Where, n is number of electrons striking the target per second
hence n=eI.t=1.6×10−195×10−3×1
therefore n=3.125×1016/s
Also v=m2eV
=(2×1.6×10−19×12×103/9.1×10−31)1/2
=6.49×107m/s
Illustration
In certain element the K electron energy is -18.525 ke V and the L electron energy is – 3 keV. When an electron jumps from the L to K shell, an X-ray photon is emitted. Calculate the wavelength of the X-rays.
Solution
Using Moseley’s law, we get
f2f1=b(Z2−a)b(Z1−a)
or c/λ2c/λ1=(Z2−a)2(Z1−a)2
or λ2=λ1(Z1−a)2/(Z2−a)2
hence λ2=200×(74−1)2/(78−1)2
or λ2=179.76A˚
Practice Questions (Basic Level)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Practice Questions (JEE Main Level)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Practice Questions (JEE Advance Level)
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
