Video Lecture
Theory For Notes Making
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Objective Assignment
1.
If a surface has a work function 4.0 eV, what is the maximum velocity of electrons liberated from the surface when it is irradiated with ultra violet radiation of wavelength 0.2 µm?
(a)4.4 × 105 m/s
(b)8.8 × 107 m/s
(c)8.8 × 105 m/s
(d)4.4 × 107 m/s.
Ans (c)
2.
The work function for Aluminium surface is 4.2 eV. The cut off wavelength for the photoelectric effect for the surface is
(a)1500 Å
(b)2955 Å
(c)3100 Å
(d)4200 Å.
Ans (b)
3.
If the frequency of light in a photoelectric experiment is doubled the stopping potential will be
(a) halved
(b) doubled
(c) more than double
(d) less than double
Ans (c)
4.
If the distance of 100 watt lamp is increased from the photo cell, the saturation current i in the photocell varies with the distance d as
(a) \displaystyle i\propto {{d}^{2}}
(b) \displaystyle i\propto d
(c) i\propto \frac{1}{d}
(d) i\propto \frac{1}{{{{d}^{2}}}}.
Ans (d)
5.
Light of wavelength 3500 Å is incident on two metals A and B whose work functions are 4.2 eV and 1.9 eV respectively. Photoelectrons will be emitted by
(a) metal A only
(b) metal B only
(c) both A and B
(d) none
Ans (b)
6.
If the speed of photoelectrons is 104 ms–1, what should be the frequency of incident radiation on the potassium metal? Work function of potassium = 2.3 eV.
(a) 8.5 × 1018 Hz
(b) 6.9 × 1018 Hz
(c) 5.5 × 1018 Hz
(d) 2.0 × 1018 Hz
Ans (b)
6.
The work function for a metal surface is 2.0 eV. Determine the longest wavelength that will eject photoelectrons from the metal surface.
(a) 6000 Å
(b) 1000 Å
(c) 4000 Å
(d) 8000 Å
Ans (a)
7.
A photosensitive metallic surface has work function hn0. If photons of energy 2hn0 falls on this surface, the electrons come out with a maximum velocity of 4 × 106 m/s. When the photon energy is increased to 5hn0, then maximum velocity of photoelectrons will be:
(a) 2 × 106 m/s
(b) 2 × 107 m/s
(c) 8 × 107 m/s
(d) 8 × 106 m/s
Ans (d)
8.
If the maximum kinetic energy of emitted photo electrons from a metal surface of work function 2.5 eV, is 1.7 eV. If wavelength of incident radiation is halved, then stopping potential will be
(a) 2.5 V
(b) 6.7 V
(c) 5 V
(d) 1.1 V
Ans (b)
9.
In a photoelectric experiment, the wavelength of incident radiation is reduced from 6000Å to 4000Å then
(a) Stopping potential will decrease
(b) Stopping potential will increase
(c) Kinetic energy of emitted electrons will decrease
(d) The value of work function will decrease
Ans (b)
10.
Figure represents the graph of kinetic energy (K) of photoelectrons (in eV) and frequency (n) for a metal used as cathode in photoelectric experiment. The work function of metal is
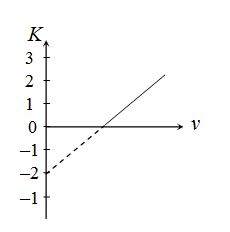
(a) 1 eV
(b) 1.5 eV
(c) 2 eV
(d) 3 eV
Ans (c)
11.
In a photoelectric effect experiment
(a) on increasing intensity and keeping frequency fixed the saturation current decreases.
(b) on increasing intensity and keeping frequency fixed the saturation current remains constant.
(c) on increasing intensity, saturation current may increase.
(d) on increasing frequency saturation current may increase.
Ans (c)
12.
When a certain metallic surface is illuminated with monochromatic light of wavelength \displaystyle \lambda , the stopping potential for photoelectric current is 3V0. When the same surface is illuminated with the light of wavelength 2 \displaystyle \lambda , the stopping potential is V0. The threshold wavelength for the surface for photoelectric effect is
(a) \frac{{4\lambda }}{3}
(b) 4 \displaystyle \lambda
(c) 6 \displaystyle \lambda
(d) 8 \displaystyle \lambda
Ans (b)
13.
The work function of aluminium is 4.2 eV. If two photons, each of energy 3.5 eV strike an electron of aluminium, then emission of electrons will be
(a) possible
(b) not possible
(c) data is incomplete
(d) depend upon the density of the surface
Ans (b)
14.
The work function for potassium is 2.25 eV. A beam with a wavelength of 400 nm has an intensity of 10-9 W/m2. Find
(a)
the maximum kinetic energy of the photoelectrons,
(a) 0.245 eV
(b) 1.045 eV
(c) 0.855 eV
(d) 10.674 eV
Ans (c)
(b)
the number of electrons emitted per meter square per second from the surface assuming 3% of the incident photons are effective in ejecting electrons.
(a) 2 x 107 m-2s-1
(b) 9 x 107 m-2s-1
(c) 13 x 107 m-2s-1
(d) 6 x 107 m-2s-1
Ans (d)
15.
The cutoff wavelength for photoemission from a material is 360 nm. What is the maximum speed of ejected electrons when it is illuminated by photons of wavelength 280 nm?
(a) 0.39 x 105 m/s
(b) 4.26 x 105 m/s
(c) 2.33 x 105 m/s
(d) 5.89 x 105 m/s
Ans (d)
16.
The threshold wavelength for cesium is 686 nm. If light of wavelength 470 nm illuminates the surface, what is the maximum speed of the photoelectrons?
(a) 5.4 x 105 m/s
(b) 10.7 x 105 m/s
(c) 4.6 x 105 m/s
(d) 3.2 x 105 m/s
Ans (a)
17.
The work function for lithium is 2.3 eV.
(a)
What is the maximum kinetic energy of photoelectrons when the surface is illuminated with light of wavelength 400 nm?
(a) 0.2 eV
(b) 0.4 eV
(c) 0.8 eV
(d) 1.5 eV
Ans (c)
(b)
If the stopping potential is 0.6 V, what is the wavelength?
(a) 428 nm
(b) 450 nm
(c) 476 nm
(d) 328 nm
Ans (a)
18.
When radiation of wavelength 350 nm is incident on a surface, the maximum kinetic energy of the photoelectrons is 1.2 eV. What is the stopping potential for a wavelength of 230 nm?
(a) 2.56 V
(b) 3.00 V
(c) 3.05 V
(d) 3.65 V
Ans (c)
Subjective Assignment
Q.1
Define ‘intensity’ of radiation in photon picture of light.
Q.2
Show on a plot the nature of variation of photoelectric current with the intensity of radiation incident on a photosensitive surface.
Q.3
The stopping potential in an experiment on photoelectric effect is 1.5V. What is the maximum kinetic energy of the photoelectrons emitted ?
Q.4
The maximum kinetic energy of a photoelectron is 3 . What is its stopping potential ?
Q5
The stopping potential in an experiment on photoelectric effect is 2V. What is the maximum kinetic energy of the photoelectrons emitted ?
Q.6
Define the term ‘stopping potential’ in relation to photoelectric effect.
Q.7
For a given photosensitive material and with a source of constant frequency of incident radiation, how does the photocurrent vary with the intensity of incident light ?
Q.8
Why is photoelectric emission not possible at all frequencies ?
Q.9
In an experiment on photoelectric effect, the slope of the cut-off voltage versus frequency of incident light is found to be 4.12 × 10–15 V s. Calculate the value of Planck’s constant.
Q.10
The work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
Q.11
Light of frequency 7.21 × 1014 Hz is incident on a metal surface. Electrons with a maximum speed of 6.0 × 105 m/s are ejected from the surface. What is the threshold frequency for photoemission of electrons?
Q.12
It is easier to remove an electron from sodium than from copper. Which metal has higher value of threshold frequency ?
Q.13
When a metal surface is exposed with a monochromatic light, then all the photoelectrons are not emitted from the metal surface with the same kinetic energy. Why?
Q.14
If an electromagnetic wave of wavelength \displaystyle \lambda is incident on a photosensitive surface of negligible work function. If the photoelectrons emitted from this surface have the de-Broglie wavelength \displaystyle {{\lambda }_{1}}, prove that \displaystyle \lambda =\left( {\frac{{2mc}}{h}} \right)\,\lambda _{1}^{2}.
Q.15
Plot a graph showing variation of stopping potential (V0) with the frequency (v) of the incident radiation for a given photosensitive material..Hence state the significance of the threshold frequency in photoelectric emission. Using the principle of energy conservation, write the equation relating the energy of incident photon, threshold frequency and the maximum kinetic energy of the emitted photoelectrons.
Q.16
(i) Define the term ‘threshold frequency’ as used in photo-electric effect.
(ii) Plot a graph showing the variation of photoelectric current as a function of anode potential for two light beams having the same frequency but different intensities, \displaystyle {{I}_{1}} and \displaystyle {{I}_{1}} \displaystyle \left( {{{I}_{1}}\,>{{I}_{2}}} \right)
Q.17
(a) Define the term ‘stopping potential’.
(b) Plot a graph showing the variation of photoelectric current as a function of anode potential for two light beams of same intensity but of different frequencies, \displaystyle {{v}_{1}} and \displaystyle {{v}_{2}} \displaystyle \left( {{{v}_{1}}\,>\,{{v}_{2}}} \right).
Q.18
Two monochromatic radiations of frequencies \displaystyle {{v}_{1}} and \displaystyle {{v}_{2}} \displaystyle \left( {{{v}_{1}}\,>{{v}_{2}}} \right) and having the same intensity are, in turn, incident on a photosensitive surface to cause photoelectric emission. Explain, giving reason, in which case (i) more number of electrons will be emitted and (ii) maximum kinetic energy of the emitted photoelectrons will be more.
Q.19
A 100W sodium lamp radiates energy uniformly in all directions. The lamp is located at the centre of a large sphere that absorbs all the sodium light which is incident on it. The wavelength of the sodium light is 589 nm. (a) What is the energy per photon associated with the sodium light? (b) At what rate are the photons delivered to the sphere?
Q.20
The threshold frequency for a certain metal is 3.3 × 1014 Hz. If light of frequency 8.2 × 1014 Hz is incident on the metal, predict the cutoff voltage for the photoelectric emission.
Q.21
Light of wavelength 488 nm is produced by an argon laser which is used in the photoelectric effect. When light from this spectral line is incident on the emitter, the stopping (cut-off) potential of photoelectrons is 0.38 V. Find the work function of the material from which the emitter is made.
Q.22
Ultraviolet light of wavelength 2271 Å from a 100 W mercury source irradiates a photo-cell made of molybdenum metal. If the stopping potential is –1.3 V, estimate the work function of the metal. How would the photo-cell respond to a high intensity ( \displaystyle \sim\,{{10}^{5}}Wm–2) red light of wavelength 6328 Å produced by a He-Ne laser?
Q.23
The work function for the following metals is given:
Na: 2.75 eV; K: 2.30 eV; Mo: 4.17 eV; Ni: 5.15 eV. Which of these metals will not give photoelectric emission for a radiation of wavelength 3300 Å from a He-Cd laser placed 1 m away from the photocell? What happens if the laser is brought nearer and placed 50 cm away?
Q.24
Blue light can eject electrons from a photosensitive surface while orange light can not. Will violet and red light eject electrons from the same surface?
Q.25
Draw a plot showing the variation of photoelectric current with collector plate potential for two different frequencies, \displaystyle {{v}_{1}}\,>\,{{v}_{2}} of incident radiation having the same intensity. In which case will the stopping potential be higher ? Justify your answer.
Q.26
(a) Ultraviolet light of wavelenth 2271 Å from a 100 W mercury source is incident on a photocell made of molybdenum metal. If the stopping potential is 1.3 V, estimate the work function of the metal.
(b) How would the photocell respond to high intensity \displaystyle \left( {{{{10}}^{5}}w/{{m}^{2}}} \right)red light of wavelength 6328 Å produced by a He–Ne laser ?
Q.27
(a) Why photoelectric effect can not be explained on the basis of wave nature of light ? Give reasons.
(b) Write the basis features of photon picture of electromagnetic radiation on which Einstein’s Photoelectric equation is based.
Q.28
(a) State three important properties of photons which describe the particle picture of electromagnetic radiation.
(b) Use Einstein’s photoelectric equation to define the terms (i) stopping potential and (ii) threshold frequency.
Q.29
The work function of caesium metal is 2.14 eV. When light of frequency 6 ×1014Hz is incident on the metal surface, photoemission of electrons occurs. What is the
(a) maximum kinetic energy of the emitted electrons,
(b) Stopping potential, and
(c) maximum speed of the emitted photoelectrons?
Q.30
Estimate the following:
(a) The number of photons emitted per second by a Medium wave transmitter of 10 kW power, emitting radiowaves of wavelength 500 m.
(b) The number of photons entering the pupil of our eye per second corresponding to the minimum intensity of white light that we humans can perceive (110–10 W m–2). Take the area of the pupil to be about 0.4 cm2, and the average frequency of white light to be about 6 × 1014 Hz.
Q.31
Monochromatic radiation of wavelength 640.2 nm (1nm = 10–9 m) from a neon lamp irradiates photosensitive material made of caesium on tungsten. The stopping voltage is measured to be 0.54 V. The source is replaced by an iron source and its 427.2 nm line irradiates the same photo-cell. Predict the new stopping voltage.
Q.32
A mercury lamp is a convenient source for studying frequency dependence of photoelectric emission, since it gives a number of spectral lines ranging from the UV to the red end of the visible spectrum. In our experiment with rubidium photo-cell, the following lines from a mercury source were used:
\displaystyle {{\lambda }_{1}}=3650{\AA},\,\,{{\lambda }_{2}}=3650{\AA},\,\,{{\lambda }_{3}}=4047{\AA},\,\,{{\lambda }_{4}}=4358{\AA},\,\,\,{{\lambda }_{5}}=5462{\AA},\,\,{{\lambda }_{6}}=6907{\AA}
The stopping voltages, respectively, were measured to be:
V01 = 1.28 V, V02 = 0.95 V, V03 = 0.74 V, V04 = 0.16 V, V05 = 0 V
Determine the value of Planck’s constant h, the threshold frequency and work function for the material.
Q.33
Light of intensity I and frequency \displaystyle \upsilon is incident on a photosensitive surface and causes photoelectric emission. What will be the effect on anode current when
(a) the intensity of light is gradually increased,
(b) the frequency of incident radiation is increased and
(c) the anode potential is increased ? In each case, all other factors remain the same. Explain, giving justification in each case.
Q.34
Sketch a graph between frequency of incident radiations and stopping potential for a given photosensitive material. What information can be obtained from the value of the intercept on the potential axis?
Q.35
A source of light of frequency greater than the threshold frequency is placed at a distance of 1m from the cathode of a photocell. The stopping potential is found to be V. If the distance of the light source from the cathode is reduced, explain giving reasons, what change will you observe in the (i) photoelectric current (ii) stopping potential ?
Q.36
In a plot of photoelectric current versus anode potential, how does
(a) the saturation current vary with anode potential for incident radiations of different frequencies but same intensity?
(b) the stopping potential vary for incident radiations of different intensities but same frequency ?
(c) Photoelectric current vary for different intensities but same frequency of incident radiations?
Q.37
What is Photoelectric effect ? Explain experimentally the variation of photoelectric current with
(a) the intensity of the incident light
(b) the potential difference between the plates and (ii) the potential difference between the plates and (iii) the frequency of the incident light and hence state the laws of photoelectric emission.
Q.38
State laws of photo electric emission. Establish Einstein photo-electric relation. Explain the laws of photo-electric emission on the basis of this relation.
Q.39
What is photoelectric effect ? Give any two practical applications of this effect. Write Einstein’s photoelectric equation and use it to explain
(a) independence of maximum energy of the emitted photoelectrons from intensity of incident light
(b) existence of a threshold frequency of the emitted photoelectrons from intensity of incident light.
Q.40
The figure shows a plot of three curves a, b, c, showing the variation of photocurrent vs collector plate potential for three different intensities \displaystyle {{I}_{1}},\,\,{{I}_{2}} and \displaystyle {{I}_{3}} having frequencies \displaystyle {{v}_{1}},\,\,{{v}_{2}} and respectively incident on a photosensitive surface. Point out the two curves for which the incident radiations have same frequency but different intensities.
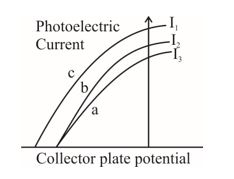
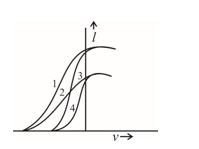
Q.41
The given graph shows the variation of photo-electric current (I) versus applied voltage (V) for two different photosensitive materials and for two different intensities of the incident radiation. Identify the pairs of curves that correspond to different materials but same intensity of incident radiation.
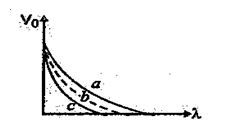
Q.42
For three different materials, the variation of the stopping potential \displaystyle {{V}_{0}} and wavelength \displaystyle \lambda of the incident light is shown by curves a, b and c in fig. Which material has maximum work function and which one has least work function?
Q.43
Fig. shows variation of stopping potential ( \displaystyle {{V}_{0}}\,) with frequency ( \displaystyle \upsilon ) for two photosensictive materials \displaystyle {{M}_{1}} and \displaystyle {{M}_{2}}.
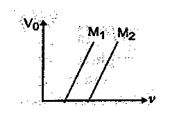
(a) Why is the slope same for both lines?
(b) For which material will the emitted electrons have greater kinetic energy for the incident radiations of the same frequency? Justify your answer.
Q.44
The graph of fig. shows the variation of photoelectric current with collector plate potential for different frequencies of incident radiations.
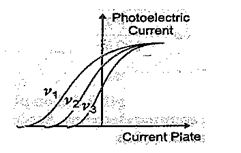
(a) Which physical parameter is kept constant for the three curves?
(b) Which frequency ( \displaystyle {{v}_{1}},\,\,{{v}_{2}}\,\,or\,\,{{v}_{3}} ) is the highest?
Q.45
In Fig. shows the variation of stopping potential \displaystyle {{V}_{0}}\, with the frequency \displaystyle \upsilon of the incident radiation for two photosensitive metals P and Q :
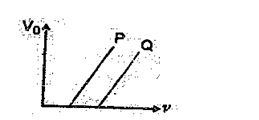
(a) Explain which metal has smaller threshold wavelength.
(b) Explain, giving reason, which metal emits photoelectrons having smaller kinetic energy, for the same wavelength of incident radiation.
(c) If the distance between the light source and metal P is doubled, how will the stopping potential change.
